There are two major defects in the application of nano-calcium carbonate to organic media: one is that nano-calcium carbonate is an inorganic material with hydrophilic and oleophobic surface. It has poor dispersion in polymers and poor affinity with organisms. It is easy to form agglomerates a, leading to material performance degradation; Secondly, nano-calcium carbonate has small particle size, a large number of surface atoms, large surface energy, strong interaction between particles, which easily forms agglomeration of nano-calcium carbonate powder. As the amount of nano-calcium carbonate used increases, these defects become more obvious, excessive filling will make the material unusable.
Stearic acid is a common long-carbon chain saturated fatty acid. It has both the lipophilic end of the long carbon chain and the hydrophilic end of the carboxyl group. The surface of nano calcium carbonate is hydrophilic, so stearic acid is coated on the nano, the surface of calcium carbonate can greatly improve its lipophilicity. When it is filled in rubber, plastics, advanced inks, its large specific surface area and high specific surface energy are beneficial to the relationship between calcium carbonate particles and organic polymer molecules. The strong bond between them can make the surface of the product bright and have excellent performance.
1. The mechanism of stearic acid coating modified nanometer calcium carbonate
In recent years, studies on coating and modifying nanometer calcium carbonate with stearic acid have also emerged in endlessly.
Chen Yijian et al. explored the formation process of stearic acid (SA) monolayer calcium carbonate crystals at the air-water interface. Using electron microscope and in-situ Brewster angle microscope for testing and characterization, it was observed that under the monolayer of stearic acid, the final calcium carbonate crystals were formed by particle precursor rather than directly derived from solvation. ion. From scanning electron microscopy (SEM) and transmission electron microscopy (TEM), it can be found that the precursor particles are uniform spheres of amorphous calcium carbonate with a diameter of less than 100 nm. The experiment is to produce calcium carbonate through the reaction of Ca(OH)2 and CO2. Amorphous calcium carbonate is produced in the early stage of mineralization, and it exists stably for at least 0.5h. As the quantity increases, amorphous calcium carbonate aggregates to form Calcite phase calcium carbonate.

Xuetao Shi et al. used commercial stearic acid to coat precipitated calcium carbonate under water phase conditions, the content of stearic acid in the coated calcium carbonate was 3% to 13.5%. Fourier infrared (FTIR), thermogravimetric (TG) and differential scanning calorimetry (DSC) analysis showed that there is no free stearic acid on the surface of calcium carbonate, only calcium stearate. It is found that the formed calcium stearate is partially chemically adsorbed and partially physically adsorbed on the surface of the coating layer, and can solve the problem that calcium carbonate cannot be fully coated on the surface under water phase conditions. The maximum coating amount is 3.25% .
2. The effect of long-chain fatty acids on calcium carbonate
Long-chain fatty acids also have important effect on the formation of calcium carbonate.
Jiuxin Jiang et al. added various long-chain fatty acids-lauric acid (lauric acid), palmitic acid (hexadecanoic acid) and stearic acid (octadecanoic acid) while blowing carbon dioxide into the calcium hydroxide suspension. Acid) to explore the formation of calcium carbonate. It was found that the addition of long-chain fatty acids did not affect the crystal form of calcium carbonate, but affected the morphology of the calcium carbonate particles produced. When lauric acid is added, the dispersibility of calcium carbonate particles is greatly improved; when a large amount of palmitic acid and stearic acid are added, a microrod-like structure and a spindle-like structure are formed. The author proposes that during the carbonization reaction of calcium hydroxide and carbon dioxide, on the one hand, the length of the carbon chain affects the shape of the micelles formed by the calcium hydroxide suspension, on the other hand, the contact mode between the micelles determines the final formation. The morphology of calcium carbonate.
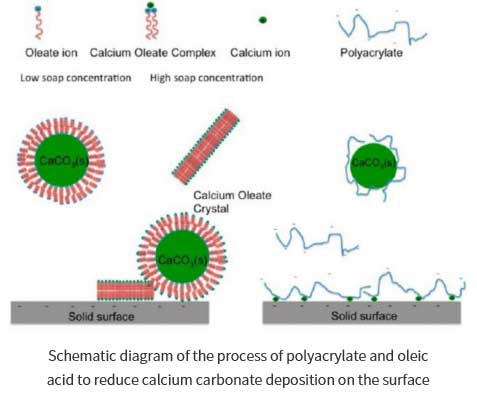
Hao Wang et al. studied the effects of cleaning agents such as polymers, fatty acids, soap liquids on the crystallization, nucleation and sedimentation of active calcium carbonate on hard surfaces (such as stainless steel and silicon surfaces). Thus, on the similar principle, it is instructed how the dishwasher can better remove oil stains during the cleaning process with detergent
3. Application of active nano calcium carbonate
Nano calcium carbonate modified by stearic acid has an important influence as a filler for organic polymers such as silicone resin and polypropylene.
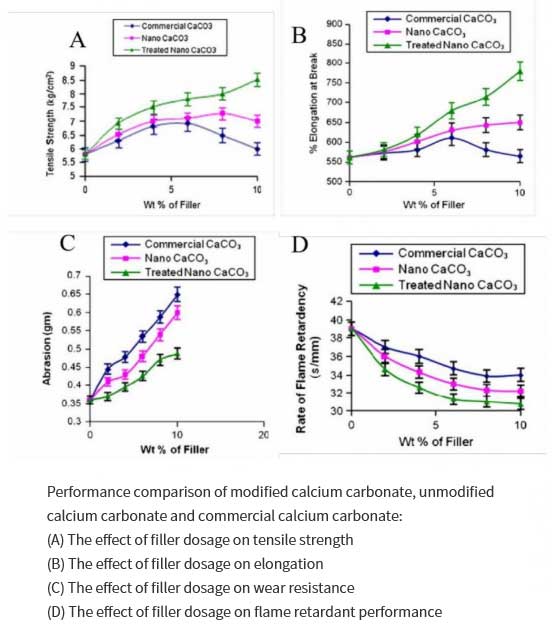
Satyendra Mishra et al. studied the effect of nano-calcium carbonate modified by stearic acid on the properties of silicone resin composites. In the presence of sodium dodecyl sulfonate, they used a certain concentration of CaCl2 and NH4HCO3 to react, filtered and dried to obtain nano calcium carbonate powder. Then in the presence of toluene, a certain amount of stearic acid and nano-calcium carbonate were stirred and mixed to obtain surface-modified nano-calcium carbonate with different stearic acid concentrations, and then added to silicone resin as a filler to improve its performance and obtain modified nano-calcium carbonate. Composite materials, the results show that compared with unmodified nano-calcium carbonate and commercial calcium carbonate, the surface-modified nano-calcium carbonate can greatly improve the tensile strength, elongation, wear resistance and flame retardancy of the composite material. Surface modification can also produce strong adhesion, which makes the polymer chain stronger and improves the thermal stability of the polymer. Based on the high strength and toughness of these nanocomposites, they can be used in cable connectors, electrical and lighting switchgear also of great value in the aerospace field.
Mahdi Rahmani et al. studied the dispersion properties of stearic acid-coated nano-calcium carbonate for polypropylene matrix. TGA was used to analyze the content of stearic acid on the surface of calcium carbonate after the actual coating, and field emission scanning electron microscopy was used to observe the dispersion performance of the sample in the organism after monolayer and multi-layer stearic acid coated nanometer calcium carbonate. The results show that the nano-calcium carbonate modified with stearic acid is filled in the polypropylene organism and can be well dispersed, which reduces the interaction between particles and the adhesion between polymers. After surface modification of stearic acid, nano calcium carbonate eliminates its hydrophilicity and greatly increases the compatibility with the polymer matrix.
As common long-chain fatty acid, stearic acid is cheap and has a wide range of uses and can well modify nano-calcium carbonate. As a cheap and easy-to-obtain filler, activated nano calcium carbonate modified by stearic acid can be well dispersed in many organisms, and can improve the mechanical properties such as tensile strength, elongation, abrasion resistance and flame retardancy of the organism and thermodynamic properties, so choosing stearic acid to modify nanometer calcium carbonate has good research and application value.
Source: Zhou Wei. Surface modification of nanometer calcium carbonate and preparation of hollow rice granular strontium carbonate and hollow fiber barium carbonate[D].
South China University of Technology, 2018.
