Calcium carbonate is an inorganic compound with a chemical formula of CaCO₃ and a melting point of 1339 ℃. It is commonly known as lime stone, limestone, stone powder, marble, scale, calcium caseinate, etc. Calcium carbonate is neutral, with slight moisture absorption, strong electrolyte and good covering power. It is basically insoluble in water and soluble in hydrochloric acid. It is one of the common materials on the earth. It is white solid in appearance and exists in rocks such as aragonite, calcite, chalk, limestone, marble and tufa. It is also the main component of animal skeleton or shell.

Physical Property
White solid, tasteless, odorless. There are two forms of amorphous and crystalline. The crystal type can be divided into orthorhombic system and hexagonal system, which are columnar or rhombic. The relative density was 2.93. It decomposes at 825 ~ 896.6 ℃ and decomposes into calcium oxide and carbon dioxide at about 825 ℃. The melting point is 1339 ℃ and 1289 ℃ at 10.7 MPa. Hardly soluble in water and alcohol. It reacts with dilute acid and gives off carbon dioxide, which is exothermic. It is soluble in ammonium chloride solution but almost insoluble in water.
Chemical Property
It boils and dissolves with dilute acetic acid, dilute hydrochloric acid and dilute nitric acid. When heated to 900 ℃ at 101.325 kPa, it decomposes into calcium oxide and carbon dioxide.
Heating calcium carbonate to 900°C under atmospheric pressure will decompose into quicklime and carbon dioxide (the method of industrial production of CO₂).
Calcium carbonate will react with dilute hydrochloric acid, and it will be effervescent to generate calcium chloride, water and carbon dioxide (the method of preparing CO₂ in the laboratory).
Excessive carbon dioxide is added to water mixed with CaCO3 to produce calcium bicarbonate solution. Calcium carbonate reacts with carbonic acid solution (rainwater) to produce calcium bicarbonate. Pour CO2 into the turbid lime water, and the precipitation disappears.
Main Categories
Classified by production methods
According to the different production methods of calcium carbonate, calcium carbonate can be divided into heavy calcium carbonate, light calcium carbonate, colloidal calcium carbonate and crystalline calcium carbonate.
- Heavy Calcium Carbonate
Heavy calcium carbonate can be made by directly crushing natural calcite, limestone, chalk, shells, etc. by mechanical methods (using Raymond mill or other high-pressure mills). Since the sedimentation volume of heavy calcium carbonate is smaller than that of light calcium carbonate, it is called heavy calcium carbonate.
Properties: white powder. Odorless and tasteless. There is no change in the exposed air, and the specific gravity is 2.710. The melting point is 1339°C. Almost insoluble in water. It dissolves in water containing ammonium salt or ferric oxide, but is insoluble in alcohol. It will boil and dissolve in dilute acetic acid, dilute hydrochloric acid, and dilute nitric acid. It is decomposed into calcium oxide (CaO) and carbon dioxide (CO₂) when heated. By using different grinding equipment, the heavy calcium carbonate with various particle sizes of 400 mesh, 500 mesh, 600 mesh, 800 mesh, 1000 mesh, 1250 mesh, 2500 mesh and 5000 mesh can be produced. Moreover, due to the special needs of papermaking, coating and pigment, the heavy calcium carbonate below 2 μm can be produced by ultrafine grinding.
- Light Calcium Carbonate
Light calcium carbonate, also known as precipitated calcium carbonate, is calcining limestone and other raw materials to produce lime (the main component is calcium oxide) and carbon dioxide, then add water to digest the lime to produce lime milk (the main component is calcium hydroxide), and then carbon dioxide is added to carbonize lime milk to generate calcium carbonate precipitation. Finally, it is prepared by dehydration, drying and grinding. Or it is prepared by the double decomposition reaction of sodium carbonate and calcium chloride to form calcium carbonate precipitation, then dehydration, drying and crushing to prepare. Because the sedimentation volume of light calcium carbonate (2.4-2.8mL/g) is larger than that of heavy calcium carbonate (1.1-1.9mL/g), it is called light calcium carbonate.
Properties: white powder. Tasteless and odorless. The specific gravity is about 2.71. Decomposes at 825~896.6℃. The melting point is 1339°C. There are two forms of amorphous and crystalline. The crystal type can be divided into orthorhombic system and hexagonal system, which are columnar or rhombic. Hardly soluble in water and alcohol. It is soluble in acid and emits carbon dioxide at the same time, showing an exothermic reaction. Also soluble in ammonium chloride solution. It is stable in the air and has a slight moisture absorption capacity.
Application: It can be used as a filler in industries such as rubber, plastics, papermaking, coatings and inks. It is widely used in the production of organic synthesis, metallurgy, glass and asbestos. It can also be used as a neutralizer for industrial wastewater, an antacid for gastric and duodenal ulcers, an antidote for acidosis, a sulfur dioxide scavenger in exhaust gas containing sulfur dioxide, a dairy cow feed additive, and an anti-sticking agent for oil felt. It can also be used as a raw material for tooth powder, toothpaste and other cosmetics.
Classified by powder particle size
Calcium carbonate product is a kind of powder. According to the average particle size (d) of calcium carbonate powder, calcium carbonate can be divided into calcium carbonate particles(d>5μm)、calcium carbonate powder (0.1μm<d≤1μm)、fine calcium carbonate (0.1μm<d≤1μm)、superfine calcium carbonate (0.02μm<d≤0.1μm)、ultrafine calcium carbonate (d≤0.02μm).
- Features of light calcium carbonate powder
a. The particle shape is regular and can be regarded as a monodisperse powder, but it can be in a variety of shapes, such as spindle shape, cubic shape, needle shape, chain shape, spherical shape, flake shape and quadrangular column shape. These different shapes of calcium carbonate can be prepared by controlling the reaction conditions.
b. The particle size distribution is narrow.
c. The particle size is small, and the average particle size is generally 1-3μm. To determine the average particle size of light calcium carbonate, the short axis particle size in the triaxial particle size can be used as the representative particle size, and then the median particle size as the average particle size. In addition to the description hereinafter, the average particle size refers to the average minor axis particle size.
- Features of heavy calcium carbonate powder
a. Irregular particle shape, polydisperse powder
b. The particle size distribution is wide.
c. The particle size is large, and the average particle size is generally 5-10μm. To determine the average particle size of ground calcium carbonate, it is necessary to determine the particle size distribution function and the function of powder phenomena such as particle settling velocity or specific surface area. As a simple method, measure the length and width of the particle projection on the electron micrograph, calculate the geometric average particle size as the apparent particle size, and then take the median particle size as the average particle size.
d. The average particle size of activated calcium carbonate is taken as the average particle size of light calcium carbonate or heavy calcium carbonate before surface modification.
Classified according to microscopic arrangement
According to the regular arrangement of the atoms and ions, calcium carbonate can be divided into crystalline calcium carbonate and amorphous calcium carbonate.
- Colloidal Calcium Carbonate (Activated Calcium Carbonate)
Also known as modified calcium carbonate, surface-treated calcium carbonate, colloidal calcium carbonate, or live calcium for short, it is prepared by surface modification of light calcium carbonate or heavy calcium carbonate with a surface modifier. Since calcium carbonate modified by a surface modifier generally has a reinforcing effect, that is, “active”, it is customary to call modified calcium carbonate as active calcium carbonate.
Properties: white, fine and light powder. A layer of fatty acid soap is adsorbed on the surface of particles, which makes CaCO3 colloidal active. The specific gravity is 1.99-2.01.
Application: as the filler of rubber, it can make the rubber have bright color, large elongation, high tensile strength and good wear resistance. It is also used as fillers in artificial leather, wire, PVC, coating, ink and papermaking industry. It can make the finished product have certain tensile strength and smooth appearance. In the production of microcellular rubber, it can be foamed evenly.
- Crystalline Calcium Carbonate
Properties: pure white, hexagonal crystalline powder. The specific volume is 1.2~1.4 ml/g. Soluble in acid, almost insoluble in water.
Application: used in toothpaste, medicine, etc. It can also be used as thermal insulation material and other chemical raw materials.
Main indexes of calcium carbonate
In recent years, the development of heavy calcium carbonate industry in China is very fast, especially the processing and application of superfine powder with the development of processing technology and processing equipment. The relevant national industry standard “industrial natural calcium carbonate” Hg / T 3249-1988 was revised in 2001, and the current version is Hg / T 3249-2001. In order to meet the requirements of market application and promote the development of regional heavy calcium carbonate, some places have issued local industry standards with higher requirements than national industry standards.
The physical and chemical indexes of heavy calcium carbonate are as follows:
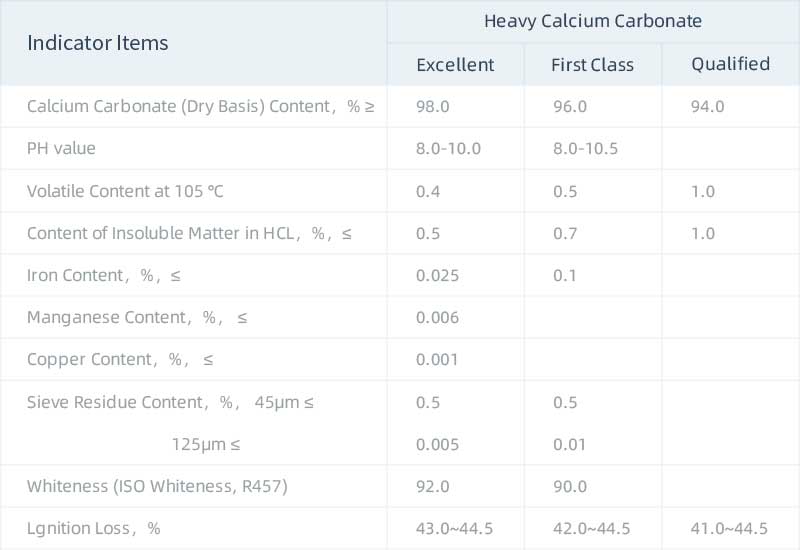
All items in the table of physical and chemical index requirements for heavy calcium carbonate are type test items. The type test is generally carried out under the following conditions: before the new product is officially put into production, stopped production for more than half a year, when the production is resumed, when the raw materials or production process change greatly, and the continuous production is conducted once every three months. Each batch of products should be inspected before leaving the factory, and can be delivered after passing the inspection. The factory inspection items of heavy calcium carbonate are as follows: calcium carbonate content, particle size, whiteness, insoluble matter in hydrochloric acid, volatile matter content at 105 ℃ and sieve residue content.
Application
Calcium carbonate has many applications, and heavy calcium carbonate is the most widely used in the construction industry.
Laboratory uses
It can also be used to make carbon dioxide in the laboratory.
Verification and determination of halogens in organic compound reactions. Water analysis. Verification of phosphorus. Decomposes silicate with ammonium chloride. Prepare calcium chloride solution to standardize soap. Manufacture of raw materials for optical neodymium glass and coating materials.
Other uses
It can be used as additive in food industry;
Commonly used in construction and paper industry;
Within 200 mesh: can be used for all kinds of feed additives, calcium content of more than 55.6, no harmful ingredients;
250 mesh to 300 mesh: used as raw material for plastic factory, rubber factory, coating factory, waterproof material factory and internal and external wall painting. The whiteness is above 85 degrees;
350 mesh to 400 mesh: used for manufacturing gusset plate, downpipe, chemical industry. The whiteness was above 93 degrees;
400 to 600 mesh: can be used for toothpaste paste, soap. The whiteness was above 94 degrees;
800 mesh: for rubber, plastic, cable, PVC whiteness above 94 degrees;
1250 mesh can be used for PVC, PE, paint, coating products, paper base coating, paper surface coating, whiteness above 95 degrees;
2500 mesh can be used in calendering film, edge banding, corrugated pipe, artificial leather, shoe material, engineering plastics, amino film plastic, wire and cable, powder coating, oily coating and other industries to improve the hardness and whiteness of materials and reduce costs;
2800 mesh is widely used in engineering plastics, wires and cables, powder coatings, oil-based coatings, calendered films, edge banding, corrugated pipes, artificial leather, shoe materials and other industries;
3000 mesh is widely used in electrical glue, powder coating, calendering film, printing ink and other industries.
Calcium carbonate can be used as a calcium supplement: the absorption rate can reach 39%, second only to calcium fruit acid, which is soluble in gastric acid, and has become the calcium supplement with the most dosage forms and most applications.
Click to view detailed application information
